Single Molecule Localisation Microscopy (SMLM)
Single Molecule Localisation Microscopy (SMLM) techniques provide invaluable information about the organisation of single molecules within a fixed cellular structure. They employ photoactivatable or photoswitchable fluorophores to localise single molecules with nanometer precision. In contrast to conventional confocal or widefield based imaging techniques where all fluorophores within your imaging plane are viewed at once, in SMLM only a small subset of fluorophores are activated at a time allowing their precise position to be recorded with a camera. Following acquisition, image reconstruction methods are employed to create a high precision map of molecule positions.
SMLM can be performed on our Nikon N-STORM system at the University of Birmingham site.
Structured Illumination Microscopy (SIM)
Structured Illumination Microscopy (SIM) can be performed on our Nikon N-SIM S at the University of Birmingham site. System specifications can be found here.
Structured Illumination Microscopy (SIM) allows the user to observe living and fixed fluorescent samples at resolution greater than a standard fluorescent microscope. Imaging at this resolution can reveal structural organisation inside a cell that can be otherwise masked.
SIM utilises a pattern of light (stripes) to excite the sample. The position and orientation of the striped pattern is rotated and the resulting emission captured. A total of fifteen images are collected (5 phases and 3 rotations) for 3D-SIM and nine images (3 phases and 3 rotations) for 2D SIM and TIRF SIM. Interference between the sample emission and the patterned excitation results in Moiré fringes. These Moiré fringes contain high-frequency information on the minute structures within the sample. The raw data is reconstructed using an algorithm to give a final super-resolved image. (115 nm in XY and 300 nm in Z depending on sample and wavelength).
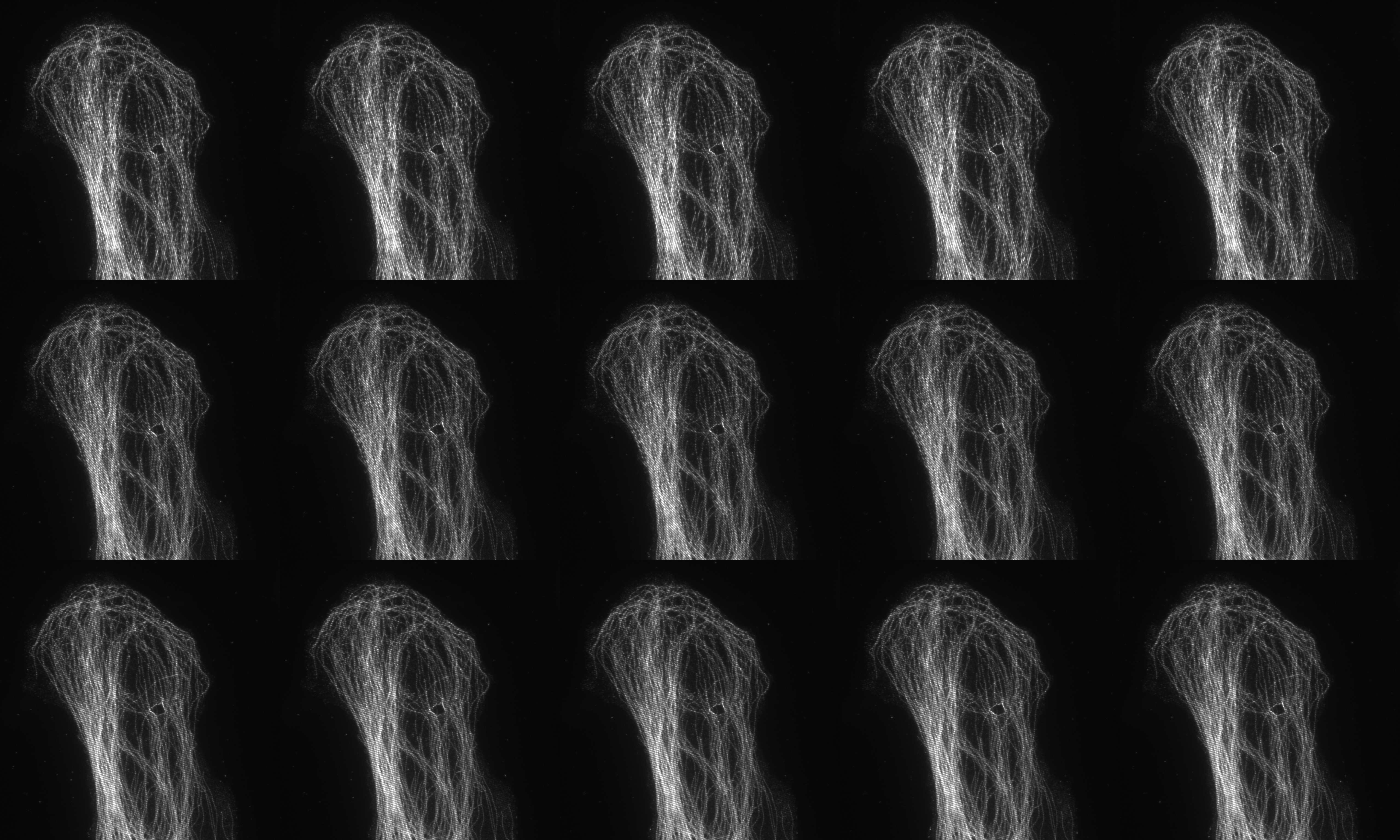
Fifteen images acquired (5 phases and 3 rotations) for 3D SIM
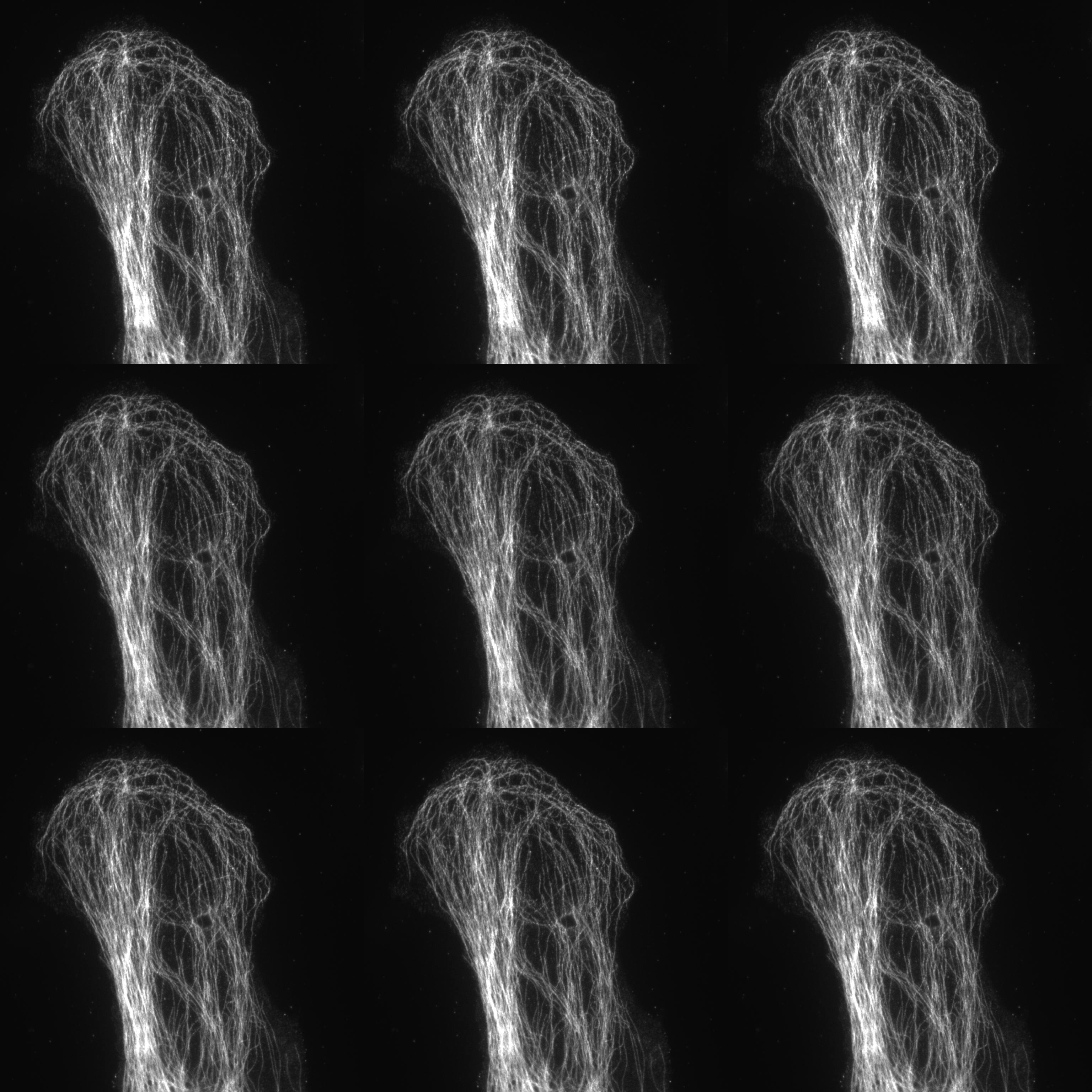
Nine images (3 phases and 3 rotations) for 2D SIM

Fluorescent tubulin microfilaments in COS7 cell image using standard widefield microscopy
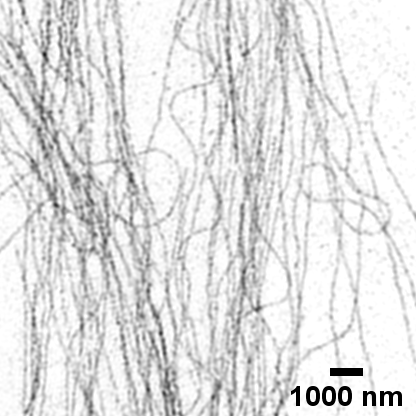
Fluorescent tubulin microfilaments in COS7 cell image using super-resolution SIM
Image courtesy of Dr Deirdre Kavanagh , COMPARE Microscopy Officer, University of Birmingham.
The SIM system is also equipped with an emission image splitter (Cairn TwinCam) and two sCMOS cameras (Hamamatsu Flash4.0). When used the system is capable of true simultaneous two colour imaging (emission filter set for green/red, red/far red and green/far red).
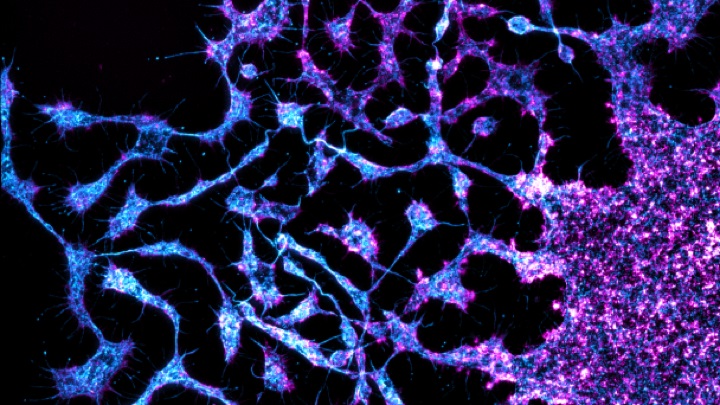
Proplatelet formation from a mature megakaryocyte
Image courtesy of Dr Steve Thomas, Senior Lecturer in Cardiovascular Sciences, University of Birmingham
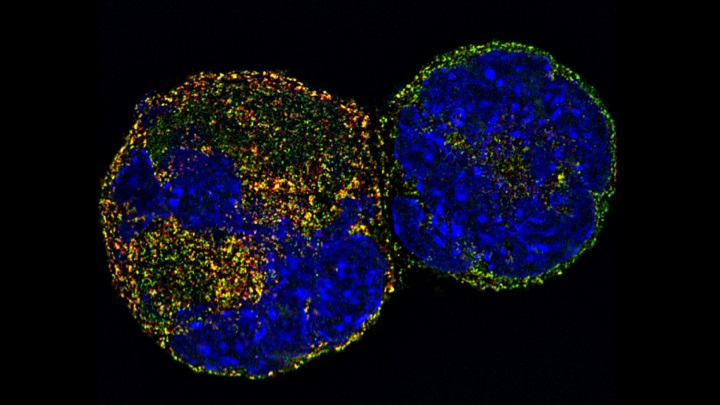
Megakaryocyte nuclei
Image courtesy of Dr Chris Smith, PDRA in Cardiovascular Sciences, University of Birmingham
Example references
Demmerle et al., Strategic and practical guidelines for successful structured illumination microscopy. Nature Protocols. 2017
Poulter et al., Platelet actin nodules are podosome-like structures dependent on Wiskott–Aldrich syndrome protein and ARP2/3 complex. Nature Communications. 2015.
Jost & Rainer. Super-resolution Multidimensional Imaging with Structured Illumination Microscopy. Annual Reviews. 2013.
NIKON N-SIM S microscope at the University of Birmingham